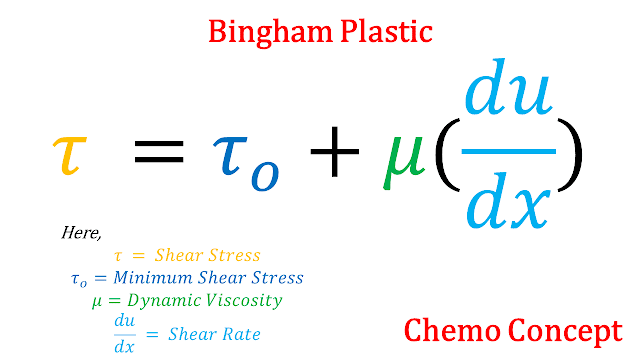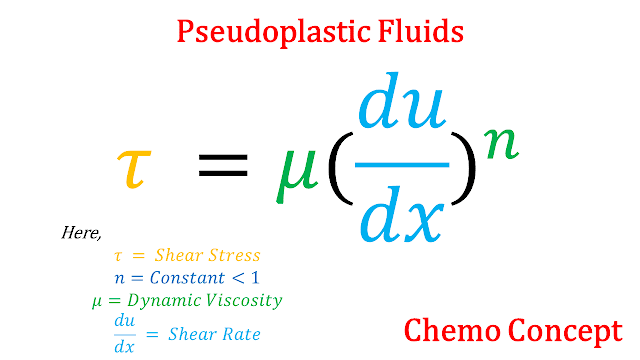- Solids
- Liquids
- Gases
So process engineers need to learn about behaviors of different phases in different conditions.
Solids have their defined shape and size. Liquids on the other side don't have any shape will take shape of the container we fill it in but have a definite volume. Gases don't have any shape or volume. Gases take the volume of the container we fill it in. Also, the behavior of that two phases is different on different pressure and temperature conditions.
As Gibbs Phase Rule Says, Any matter can be in any one, two, or all three phases simultaneously according to pressure and temperature conditions.
Solids are divided into two sub-categories
- Rigid Body - Rigid bodies don't deform on the application of force.
- Deformable Body - Deformable bodies deform on the application of force.
Another important phase is fluid which contains both liquid and gas.
What is Fluid
Let's define a fluid by formal definition: Fluid is a substance that doesn't resist distortion. This statement means when we apply force on a block of fluid. Fluid will start to distort (Shape Change).
Fluid will get divided into layers that slide on one another. Until force is applied, Layers keep sliding infinitely. As force is removed, Layers will stop and will not come to their original shape. So fluid will get permanent distortion on the application of force no matter how negligible force's magnitude is.
Liquids and Gases are included in fluid.
Types of Fluid
Different types of fluids behave differently in different conditions. Density and Viscosity are two important property which helps us distinguish between different types of fluid.
According to density, Fluids can be divided into two types:
- Incompressible Fluids: Incompressible fluids are fluids which density changes negligibly on the application of moderate temperature and pressure changes. Liquids come under these categories. Examples are Water, Milk, Acetone, Ethanol, etc.
- Compressible Fluids: Compressible fluids are fluids which density changes appreciably on the application of moderate temperature and pressure changes. Gases have come under this category. Examples are Hydrogen, Nitrogen, Helium gases, etc.
According to viscosity, Fluids can be divided into two main categories
1. Newtonian Fluids: Newtonian fluids are fluids of which follow newton's law of viscosity. Newton's law of viscosity states that the rate of shear in a fluid is directly proportional to shear stress.
Here the proportionality constant is dynamic viscosity.
2. Non-Newtonian Fluids: Non-Newtonian fluids are fluids that don't follow Newton's law of viscosity. In Non-Newtonian fluids, There are two subcategories:
a. Bingham Plastic: Bingham plastic is a special type of fluid that follows newton's law of viscosity when shear stress is applied is greater than minimum shear stress. The formula for Bingham plastic is
b. Psuedoplastic Fluids: Psuedoplastic is a special type of fluid that follows given relation
In pseudoplastic as stress increases, Viscosity will decrease. An example of pseudoplastic is blood.
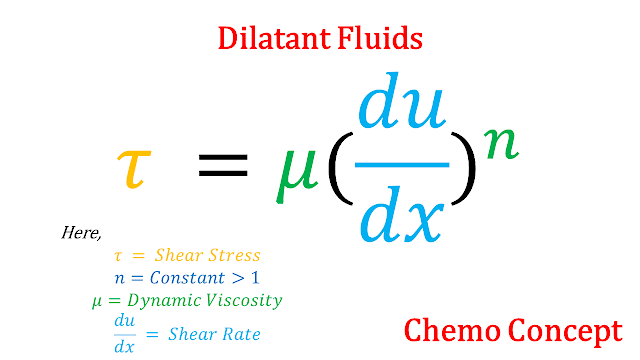
c. Dilatant Fluids: Dilatant is a special type of fluid that follows given relation
In dilatant fluids as stress increases, Viscosity will increase. An example of dilatant fluid is Sand in water.
Other special types of fluids of which viscosity depends on time also are:
1. Rheopectic Fluids: When shear stress of constant intensity is applied on fluid, Its viscosity increases with time. An example of rheopectic fluid is lubricants.
2. Thixotropic Fluids: When shear stress of constant intensity is applied on fluid, Its viscosity decrease with time. An example of thixotropic fluid is ketchup.